Processes and pathways of the water cycle
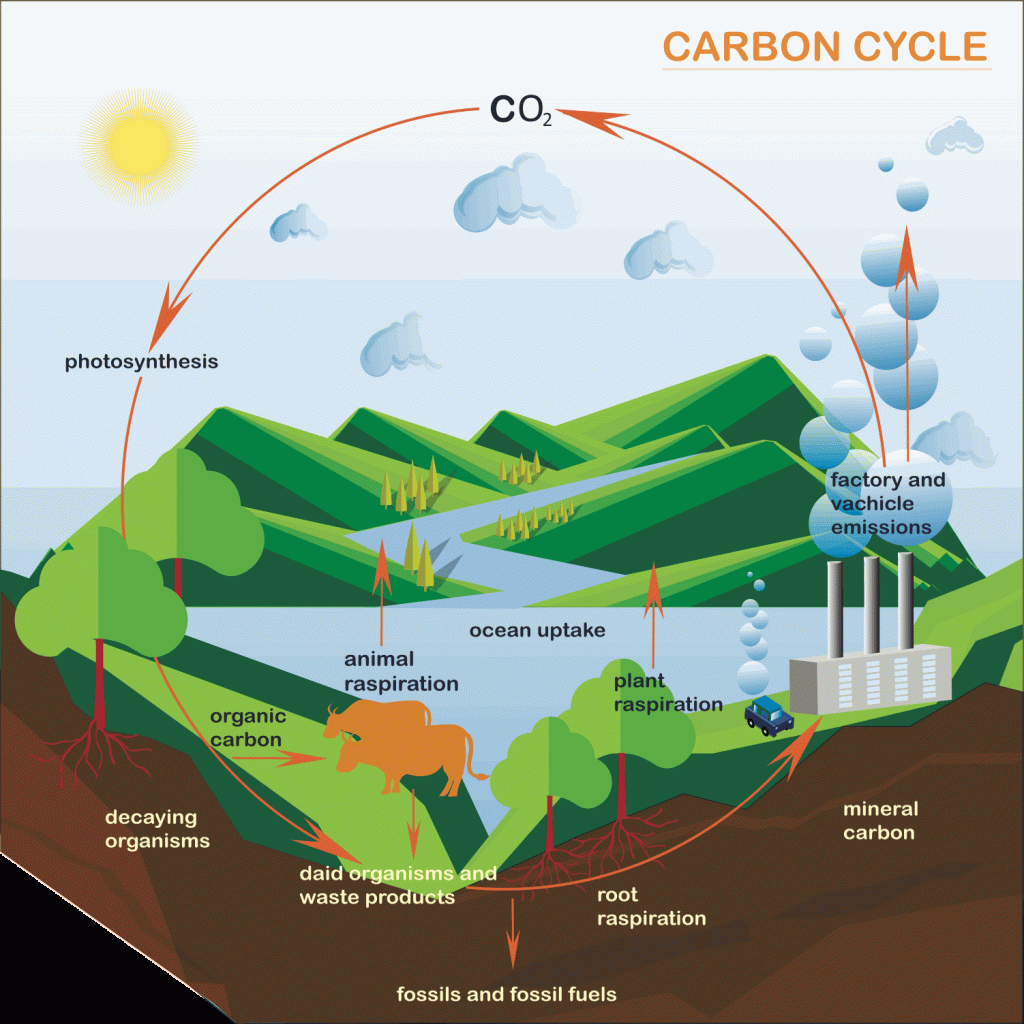
Flows between carbon stores are known as exchanges or fluxes.
Precipitation
Commonly known as acid rain, carbon dioxide in the atmosphere can mix with rainwater to form a weak carbonic acid. When precipitation happens carbon flows back to the land and oceans. As CO2 concentrations in the atmosphere are increasing due to human actions there has been an increase in the acidity of rain. This has led to an increase in the acidity of ocean water.
Photosynthesis
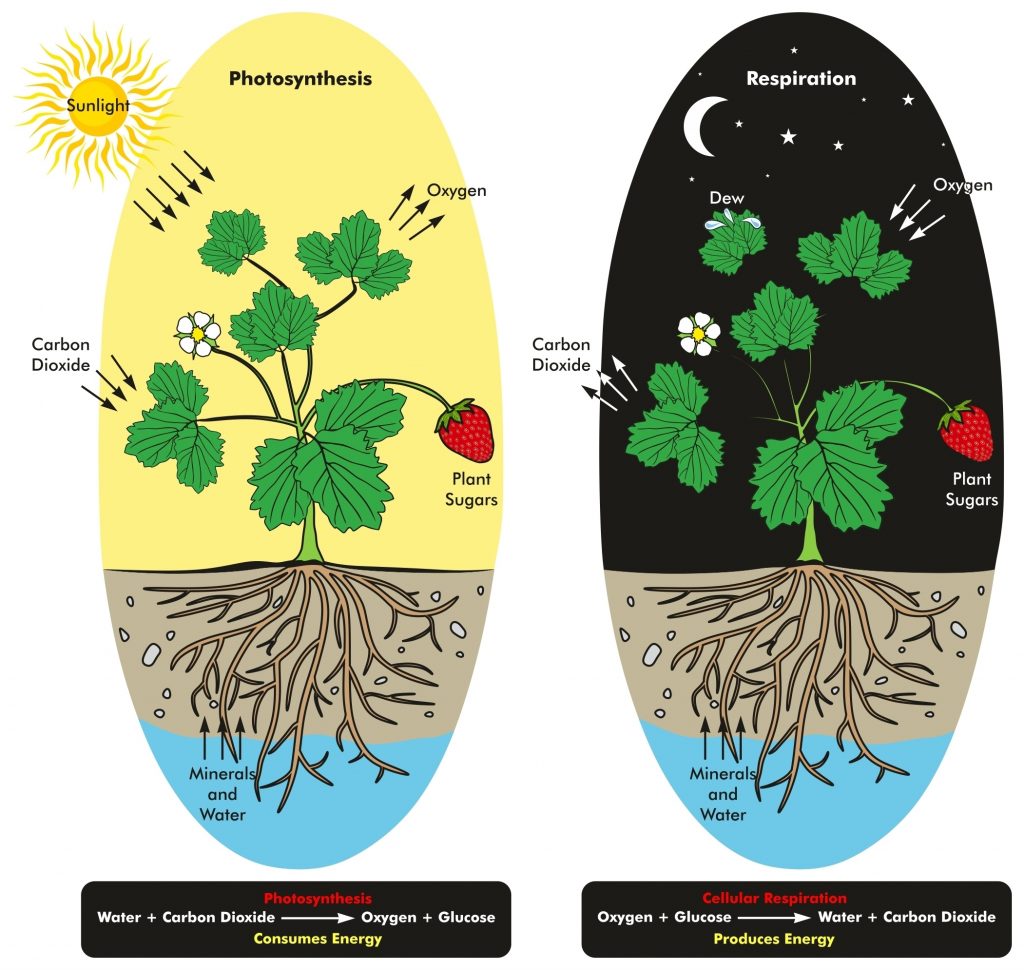
Photosynthesis is a process used by land plants and marine phytoplankton convert light energy into chemical energy that can later be released to fuel the organisms’ activities (energy transformation). This chemical energy is stored in carbohydrate molecules, such as sugars, which are manufactured from carbon dioxide and water.
Glucose is used:
• as a building block of cell walls in the form of cellulose, an important carbon store
• for respiration inside plant cells, this provides energy
• to be converted into fats and oils which act as an energy store
Respiration
Respiration is the reverse of photosynthesis. It releases energy by breaking sugars. CO2 is released into the atmosphere. Respiration and photosynthesis are elements of the fast carbon cycle. A thousand times greater volume of carbon is exchanged than is through the slow carbon cycle.
Decomposition
Carbon is released as CO2 when living organisms dies and are decomposed by microorganisms such as fungi and bacteria. The temperature has a significant impact on the rate of decomposition. Humid environments such as the tropical rainforest have rapid rates whereas colder environments such as arctic tundra have slow rates of decomposition.
Burial and Compaction
Burial and compaction is where organic matter is buried by sediment and becomes compacted. Over millions of years, this organic sediments containing carbon may form hydrocarbons such as coal and oil. Shelled organisms and coral absorb carbon dioxide from the water and converts it into calcium carbonate which builds their shells. When these organisms die they accumulate on the sea bed. Carbon dioxide is released as the carbonates dissolve. The remainder becomes compacted, forming limestone which stores carbon for millions of years.
Combustion
When organic material burns combustion occurs. This causes carbon to be released as CO2. Combustion happens naturally by wildfires caused by lightning strikes. This leads to increased nutrient and carbon recycling and results in growth being stimulated. This is essential to a number of ecosystems including grasslands in East Africa.
Human activities cause a significant amount of combustion. Burning fossil fuels such as coal, oil and gas results in stored carbon being released in CO2. This leads to a net increase in CO2 in the atmosphere. Burning biomass fuel is regarded as being carbon neutral because new crops have been planted and are absorbing CO2 from the atmosphere when plant-based materials are being burned. As long as the system is sustainably managed there is no net increase in CO2.
Carbon Sequestration
Carbon sequestration is the term used to describe the transfer of carbon from the atmosphere to plants, soil, oceans and rock formations. Carbon sequestration is both a human and natural process. Carbon capture is a term used to describe the use of technology capturing carbon emitted from power stations.
Weathering
Weathering involves the breakdown of rocks, in situ, close to or on the surface of the Earth. Weathering occurs by physical, biological or chemical processes. Chemical weathering typically involves rainwater that is weak carbonic acid (acid rain). CO2 gas in the atmosphere dissolves and CO2 is absorbed from the soil as the water flows through it. Carbonate rocks such as limestone are weathered through carbonation or solution weathering. As the rock comes into contact with acidic water it reacts and changes the calcium carbonate into calcium bicarbonate which is soluble and dissolves. Running water carries it away. 0.3 billion tonnes of carbon are transferred from rocks to the atmosphere and oceans every year.
Physical weathering widens cracks in rocks allowing more water to access the rock. Also, physical weathering results in small pieces of rock being broken off which results in them having a greater surface area that can be attacked by chemical weathering.


You must be logged in to post a comment.